🏾 Contents :
◉ Définition
TFA or Trifluoroacetic Acid is an organofluorine compound (contains a carbon-fluorine bond) with the chemical formula C2HF3O2 . It is a strong carboxylic acid due to the presence of three fluorine atoms, which are strongly electronegative. Compared to acetic acid, trifluoroacetic acid is approximately 100,000 times more potent.
Trifluoroacetic acid was discovered by Swarts in 1922, and is widely used in organic synthesis as a solvent or as an acid catalyst for various organic transformations.
TFA, discovered at the beginning of the 20th century, is prepared industrially by the electrofluorination of acetyl chloride or acetic anhydride, followed by the hydrolysis of the resulting trifluoroacetyl fluoride.
TFA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAS number | 76-05-1 | ||||||||
| Molecular mass | 114.0233 g/Mol | ||||||||
| Formula | C2HF3O2 | ||||||||
| PKa | 0.3 | ||||||||
| Synonyms | Perfluoroacetic acid, Trifluoracetic acid, Trifluoroethanoic acid | ||||||||
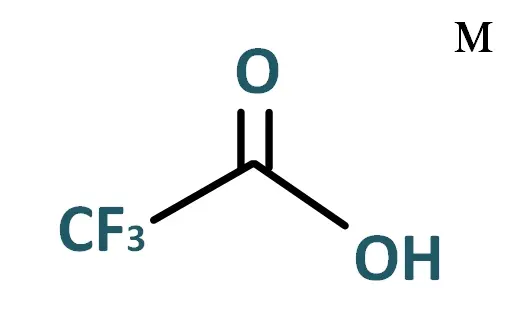
TFA : C2HF3O2
◉ Applications and uses of trifluoroacetic acid
Trifluoroacetic acid is mainly intended for the production of new pesticides, drugs and dyes, and also has great potential for application and development in the fields of materials and solvents.
Many chemical transformations must be performed using TFAs, including rearrangements, functional group deprotections, oxidations, reductions, condensations, hydroarylations, and trifluoromethylations.
◉ Solvent
- The high dielectric constant, miscibility with water and most organic solvents of TFA have allowed its use, alone or in mixture, as a solvent for various purposes. As a solvent, trifluoroacetic acid is a kind of excellent solvent for fluorination, nitration and halogenations.
- Compared to water as a solvent, trifluoroacetic acid has better solubility for organic substrates.
- For example, when the quinolone is catalyzed for hydrogenation in a common solvent, the pyridine ring is preferentially hydrogenated; on the contrary, the benzene ring will be preferentially hydrogenated in the presence of trifluoroacetic acid as solvent.
◉ In chromatography
- At low concentrations, TFA is used as an ion-pairing agent in liquid chromatography of organic compounds, especially peptides and small proteins.
- TFA is often used in high-throughput purification labs to control the peak shape of basic compounds by ensuring that residual silanols ubiquitous in silica-based C18 columns are in their neutral charge state
◉ Rearrangements
- During certain reactions there is a potential for profound structural changes to occur. Many of these structural rearrangements are triggered by intermediates involving positively charged or electron-deficient atoms.
TFA is a common catalyst for most acid-catalyst rearrangements, having the advantage of easy removal by evaporation during processing due to its low boiling point.
◉ Removal of protecting groups
- TFA is commonly used as a strong acid to remove side chain protecting groups derived from t-butyl in Fmoc peptide synthesis and in other organic syntheses to remove the t-butoxycarbonyl protecting group.
- It has also been used as the reagent of choice for the removal of nitrogen and oxygen protecting groups by solvolysis under aqueous or anhydrous conditions.
◉ Properties of Trifluoroacetic Acid
It is a colorless, volatile fuming liquid with an odor similar to that of acetic acid. It is hygroscopic and has a stimulating smell. It is miscible with water, fluorinated alkanes, methanol, benzene, ether, carbon tetrachloride and hexane. It can partially dissolve an alkane with more than six carbons as well as carbon disulfide.
TFA is a stronger acid than acetic acid, having an acid ionization constant, Ka, which is about 34,000 times higher, because the highly electronegative fluorine atoms and the attracting nature resulting electron from the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing greater acidity) and stabilizes the anionic conjugate base.
It is stable at a temperature above 205℃ stable. But its ester and amide derivatives are easily subject to hydrolysis, which allows them to prepare carbohydrates, amino acids and peptide derivatives in acid or anhydride form. It dehydrates easily under the action of phosphorus pentoxide and is transformed into trifluoroacetic anhydride.
It is common to remove excess TFA by evaporation because other reagent removal methods are often affected by this organic acid.