Summary :
◉ Definition
Antibiotic susceptibility testing (AST) is an in vitro test that determines the sensitivity or resistance of bacteria to specific antibiotics. It is performed by exposing the microorganism to different antibiotics in a controlled laboratory environment to assess their effectiveness in inhibiting or killing the bacteria.
The results of antibiotic susceptibility testing help clinicians choose the most effective antibiotic(s) to treat a bacterial infection. AST also plays a crucial role in monitoring bacterial resistance patterns. By assessing the susceptibility or resistance of bacteria to various antibiotics, it provides valuable data on the prevalence and trends of antibiotic resistance in a particular population or geographical area.
◉ Antibiotic susceptibility testing methods
There are multiple methods and techniques available for performing antibiotic susceptibility testing:
- Disc diffusion method (Kirby-Bauer method) : Antibiotic discs are placed on an agar plate with bacteria. The zone of inhibition around each disc is measured and compared to interpretive criteria.
- Broth microdilution method : Serial dilutions of antibiotics are prepared in microtiter plates. Bacterial suspension is added, and the MIC is determined as the lowest concentration inhibiting visible growth (Minimum Inhibitory Concentrations (MIC)).
- Etest method : Strips with a gradient of antibiotic concentrations are placed on agar plates (MIC strips (Etest))
- Automated systems : Bacterial growth or inhibition is measured using optical or colorimetric methods, with results interpreted by software.
- Molecular methods : PCR or DNA sequencing detects specific resistance genes or mutations.
◉ Principle of Disc diffusion method
The principles of antibiotic susceptibility testing are based on the assessment of the ability of antibiotics to inhibit the growth of bacteria or kill them.
- A standardized inoculum of bacteria (usually 0.5Mcf) is dabbed onto the surface of a dish of Mueller-Hinton (MH) agar
- Filter paper discs impregnated with antimicrobial agents are placed on the agar.
- After overnight incubation, the diameter of the zone of inhibition is measured around each disc.
- By referring to the tables of the CLSI standard / EUCAST, we obtain a qualitative report of sensitive (S), intermediate (I) or resistan (R).
◉ Culture media for antibiotic Susceptibility Testing
The culture medium must allow the growth of many bacteria and it must not contain antibiotic inhibitors :
- A pH too acidic increases the activity of ß-lactams, an alkaline medium favors aminoglycosides and macrolides, it must be between 7.2 and 7.4, a value which allows good bacterial growth and which achieves a compromise for the activity of antibiotics.
- The calcium and magnesium content must be controlled, because excess divalent cations (Ca2+, Mg2+) inhibit the action of polymyxins.
◍ The medium used for the majority of bacterial species is Mueller-Hinton agar (plus 5% blood for fastidious germs) :
- It shows acceptable lot-to-lot reproducibility for susceptibility testing.
- It is low in inhibitors which affect sulfonamide, trimethoprim and tetracycline susceptibility test results.
- It supports satisfactory growth of most pathogens.
- A large amount of data and experience has been collected on sensitivity tests carried out with this medium.
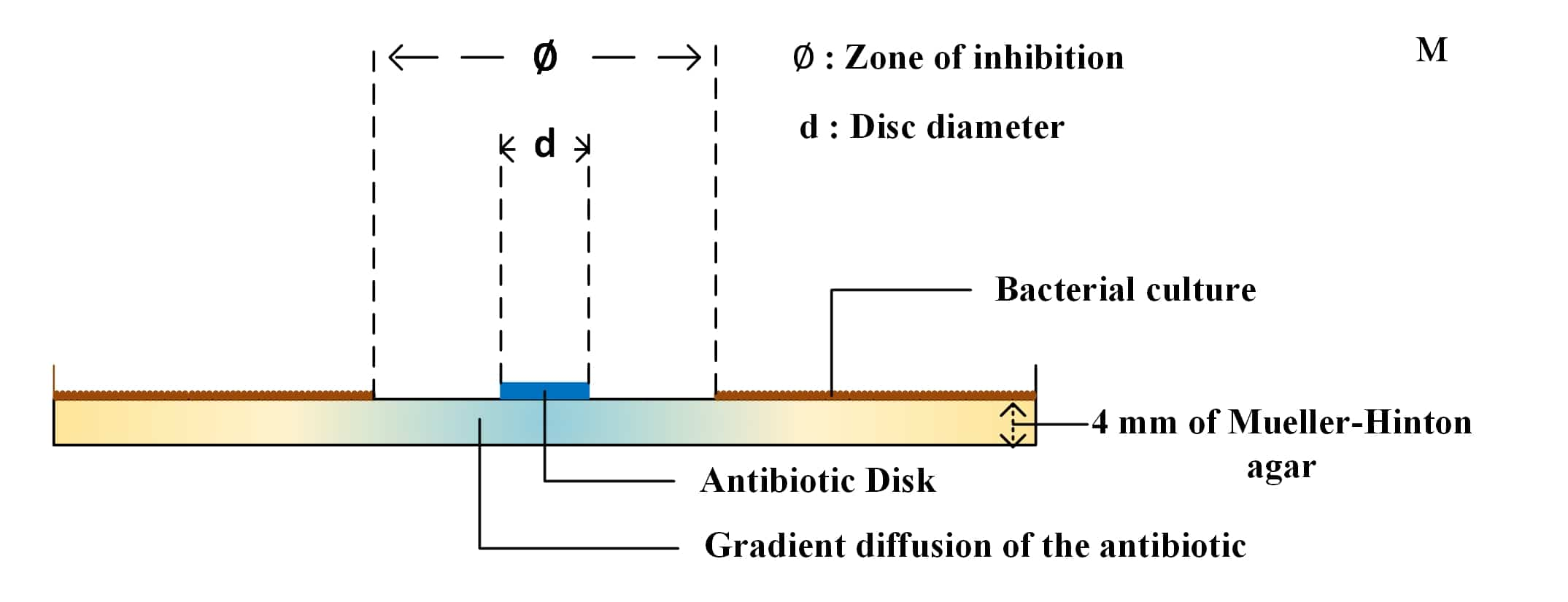
◍ It must be poured into Petri dishes to a thickness of 4 mm and the agars must be dried before use.
NOTE:
Some fastidious species, such as Haemophilus spp., Neisseria gonorrhoeae, Neisseria meningitidis, Streptococcus pneumoniae and viridans and β-hemolytic streptococci do not grow sufficiently on unsupplemented MH-agar (require supplements or different media).
If, just before use, excess surface moisture is present on the plates, place them in an incubator (35°C) or laminar flow hood at room temperature with the lids ajar until excess surface moisture is removed by evaporation (usually 10 to 30 minutes).
◉ Antibiotic discs
◍ Antibiotic discs are made from premium quality paper towels impregnated with antimicrobial agents in precise concentrations. They are clearly identified by an acronym, comprising 1 to 3 letters, printed on each side of the disc
◍ The disc cartridges must be stored in their container between +2 and +8°C in a dry place. The discs must be brought to room temperature. Any opened cartridge must be used within five days.

| Acronym | Antibiotic | Family | Disk Load (µg) |
|---|---|---|---|
| AM | Ampicillin | Aminopenicillin | 10 |
| ATM | Aztreonam | Monobactam | 30 |
| GM | Gentamicin | Aminosides | 10 |
| ETP | Ertapenem | Carbapenem | 10 |
◍ More disc abbreviations
◍ The antibiotic contained in the disc dissolves in the water of the agar. Its distribution can be schematically presented in two stagess:
- A vertical diffusion (in depth) in the medium contained in the cylinder delimited by the pre-impregnated disc
- A horizontal diffusion (laterally) which distributes the antibiotic according to a gradient of concentrations whose maximum is located at the level of the disc
◉ Inoculum size '0.5 McFarland'
The cell suspension must be prepared in sterile physiological water from a young and pure culture on an appropriate isolation medium. In the case of Neisseria gonorrhoeae, the suspension is prepared in sterile phosphate buffer at pH 7.2.
It must be adjusted using a photometer or by comparison with an opacity standard (McFarland scale). The antibiogram by diffusion is carried out with a suspension calibrated at 0.5 MF or at an DO of 0, 08 - 0.10 read at 625 nm containing approximately 108 bacteria per ml. This number is increased to 109 for Helicobacter pylori equivalent to McFarland standard 3.

☰ Preparation of the 0.5 McFarland turbidity standard
| 1 | Add 0.5 mL of a 0.048 mol/L solution of BaCl2 (1.175% w/v BaCl2 2H2O) to 99.5 mL of a 0.18 mol/L (0.36 N) solution of 2 S0 4(1% v/v) and shake vigorously. |
|---|---|
| 2 | Check the density of the suspension using a spectrophotometer with a 1 cm beam and matching cuvettes. The absorbance at 625 nm should be between 0.08 and 0.13. |
| 3 | Distribute the suspension in tubes of the same size as those used to adjust the inoculum. Seal the tubes. |
| 4 | Once sealed, store these tubes at room temperature and protect from light. Before use, mix the tube vigorously using a Vortex (6 months storage). |
◉ Antibiotic susceptibility testing seeding
☰ The inoculation must be done within 15 minutes of preparing the inoculum. It is carried out by swabbing or by flooding in such a way as to have distinct but contiguous colonies after incubation.
- 1. Immerse the swab in the suspension and remove excess liquid by swirling the swab against the sides of the tube.
- 2. Rub the entire surface of the agar dish three times, rotating the dish approximately 60°C between streaks to ensure even distribution. To minimize aerosols, avoid hitting the sides of the plate. Finally, run a swab around the edge of the agar to remove any excess moisture.
◉ Disc Application
- 1. Apply disks to the agar surface with a dispenser or manually with sterile tweezers (maximum 6 disks on a 9 cm diameter Petri dish).
- 2. Apply light pressure with tweezers or a sterile needle to ensure full contact of the disc with the agar (some dispensers do this automatically).
- 3. Rapid incubation within 15 minutes following the deposit of the discs (beyond 30 minutes the zones of inhibition will be falsely enlarged).

NOTE : Because some of the drug diffuses almost instantaneously, a disc should not be moved once it has contacted the agar surface. Instead, place a new disc in another location on the agar..
Antibiogram incubation:
Turn the Petri dish upside down and ideally incubate them within 15 min after depositing the discs, without exceeding 30 min. If they are left at room temperature after depositing the discs, the pre-diffusion of the antibiotics will generate falsely enlarged zones of inhibition.
◉ Antibiotic susceptibility testing reading
◍ After 16-18 hours of incubation, examine each plate. If the plate has been streaked satisfactorily and the inoculum concentration is correct, the resulting zones of inhibition will be uniformly circular and there will be a lawn of confluent growth.
If individual colonies are apparent, the inoculum concentration was too light and the test should be repeated.

Individual colonies are apparent

Confluent growth
◍ Measure the diameters of the zones of complete inhibition (as judged by the naked eye), including the diameter of the disc (6mm). Measure the areas to the nearest whole millimeter, using sliding calipers or a ruler.
◍ Compare the measured inhibition diameter (measured ∅) and the critical diameters d(CCsup) and D(CCinf) according to CLSI "Clinical & Laboratory Standards Institute" or EUCAST
| ∅ measured ≥ D(CCinf) | Susceptible (S) |
|---|---|
| ∅ measured < d(CCsup) | Resistant (R) |
| d(CCsup) ≤ ∅ measured < D(CCinf) | Intermediate (I) |

Antibiogram reading