Content :
- Definition
- Principle of Gram staining
- Composition and preparation of dyes
- Procedure of Gram staining
- Quality controlt
- Comments and Tips
- Gram-positive and Gram-negative bacteria
◉ What is a Gram stain ?
Gram stain is the most important and widely used microbiological differential stain, published by Hans Christian Gram in 1884, it allows bacteria to be differentiated according to 2 main criteria : their shape and their affinity for dyes :
- Shape : Pairs, Tetrads, Groups, Chains, Lancet-shaped ...
- Affinity for dyes : Gram positive or Gram negative
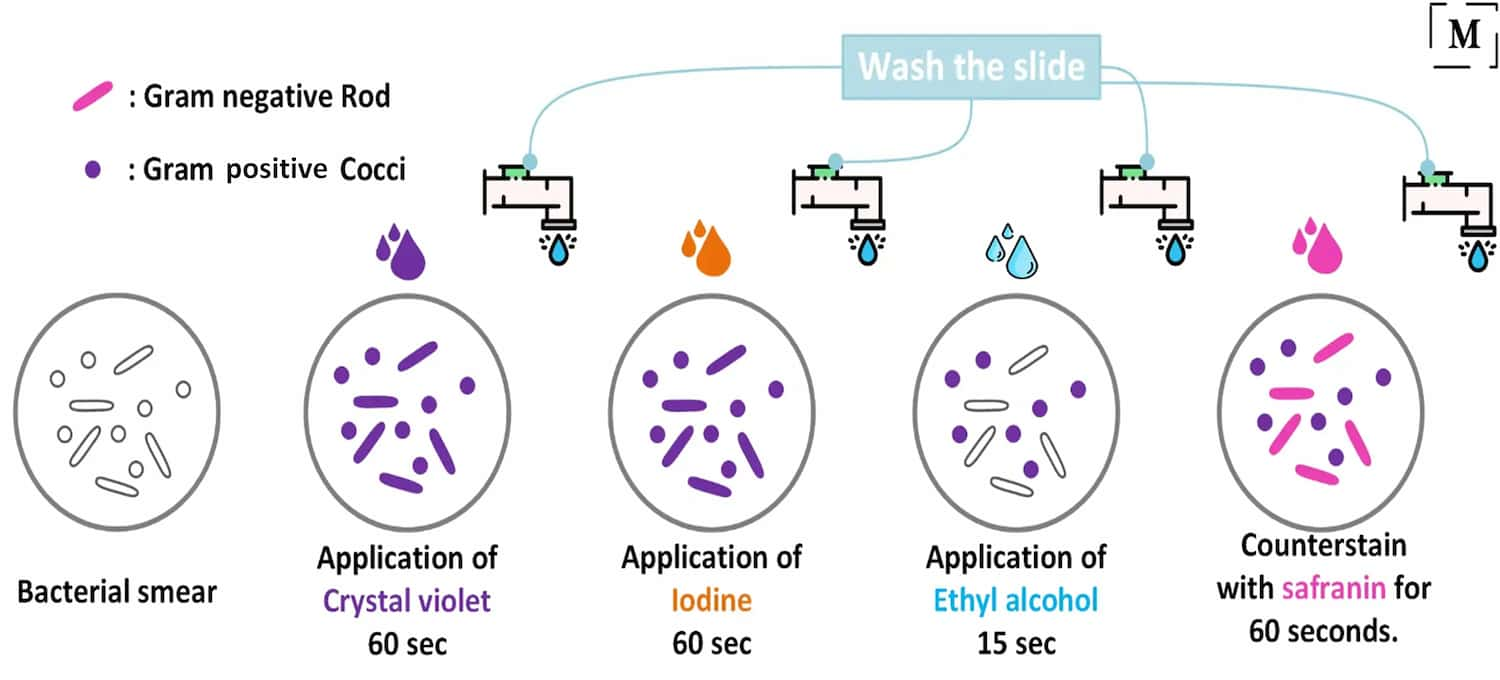
Gram staining procedure
◉ Principle of Gram staining
What makes some cells retain crystal violet (Gram positive) while others readily release the stain when alcohol is added (Gram negative)?
- 🔳 Gram-positive bacteria : Bacteria with a thick, highly cross-linked layer of peptidoglycan (20 to 80 nm) trap the primary stain-mordant complex and stain purple, and are designated Gram positive
- 🔳 Gram-negative bacteria : bacteria that have a thin layer of peptidoglycan (1 to 3 nm) with a lower percentage of cross-linkage, followed by a thin second layer called the outer membrane (7 to 8 nm), do not retain the primary stain-mordant complex upon alcohol treatment. These bacteria with a thin cell wall are counterstained with safranin and are labeled as the Gram-negative bacteria.
Both Gram-positive and Gram-negative staining of bacteria reveal the overall cell morphology so that the cells can also be further labeled as rods or cocci.
◉ Composition and preparation of Gram stain
➀ Primary Stain : Crystal Violet Staining Reagent
- Solution A "Crystal violet staining reagent" : Dissolve 20 g crystal violet (85% dye) in 100 ml of 95% ethanol.
- Solution B "Oxalate stock" : Dissolve 1 g ammonium oxalate in 100 ml water.
Solution de travail : Diluer la solution A 1:10 avec de l'eau distillée et mélanger avec la solution B 4 vol. Conserver jusqu'à 6 mois dans une bouteille en verre à température ambiante
➁ Mordant: Gram's Iodine : Iodine, 1.0 g, Potassium iodide, 2.0 g, Distilled water, 300 ml
Grind the iodine and potassium iodide in a mortar and add water slowly with continuous grinding until the iodine is dissolved. Store in amber bottles.
➂ Decolorizing Agent : Some professionals prefer an acetone decolorizer while others use a 1:1 acetone and ethanol 95% mixture.
➃ Counterstain : Mix 2.5 g safranin O with 100 ml of 95% ethanol.
Note : Some laboratories use safranin as a counterstain; however, basic fuchsin stains gram-negative organisms more intensely than safranin. Similarly, Hemophilus spp., Legionella app, and some anaerobic bacteria stain poorly with safranin.
◉ How is gram staining performed ?
🔳 Whatever the dye used, the technique remains essentially the same as follows:
- Flood the air-dried, heat-fixed smear for 30 to 60 seconds with crystal violet staining reagent. Please note that the quality of the smear (too heavy or too light cell concentration) will affect the staining results.
- Wash the blade in a gentle, indirect stream of tap water for 2 seconds.
- Flood with the mordant: iodine or lugol and wait 1 minute.
- Wash the blade in a gentle, indirect stream of tap water for 2 seconds.
- Flood the blade with decolorizing agent and wait 15 seconds or add drop by drop to release decolorizing agent.
- Flood the slide with counterstain, 'safranin' and wait 30 seconds to 1 minute.
- Wash the slide in a soft, indirect stream of tap water until no color appears in the effluent, then dry with paper towel. Be sure the bottom of the slide is clean.
- Observe the results of the staining procedure under oil immersion. Examine under the microscope, x100 objective
Note : 95% ethanol is the slowest acting solution (10 to 20 sec) and, thus, the most forgiving, it is suggested for beginners. Once Gram staining becomes routine, one may use the intermediate acting solution of 95% ethanol with acetone, which decolorizes in ∼7 to 10 sec ( acetone can be used as the decolorizer with a 1- to 2-sec reaction time). (2)
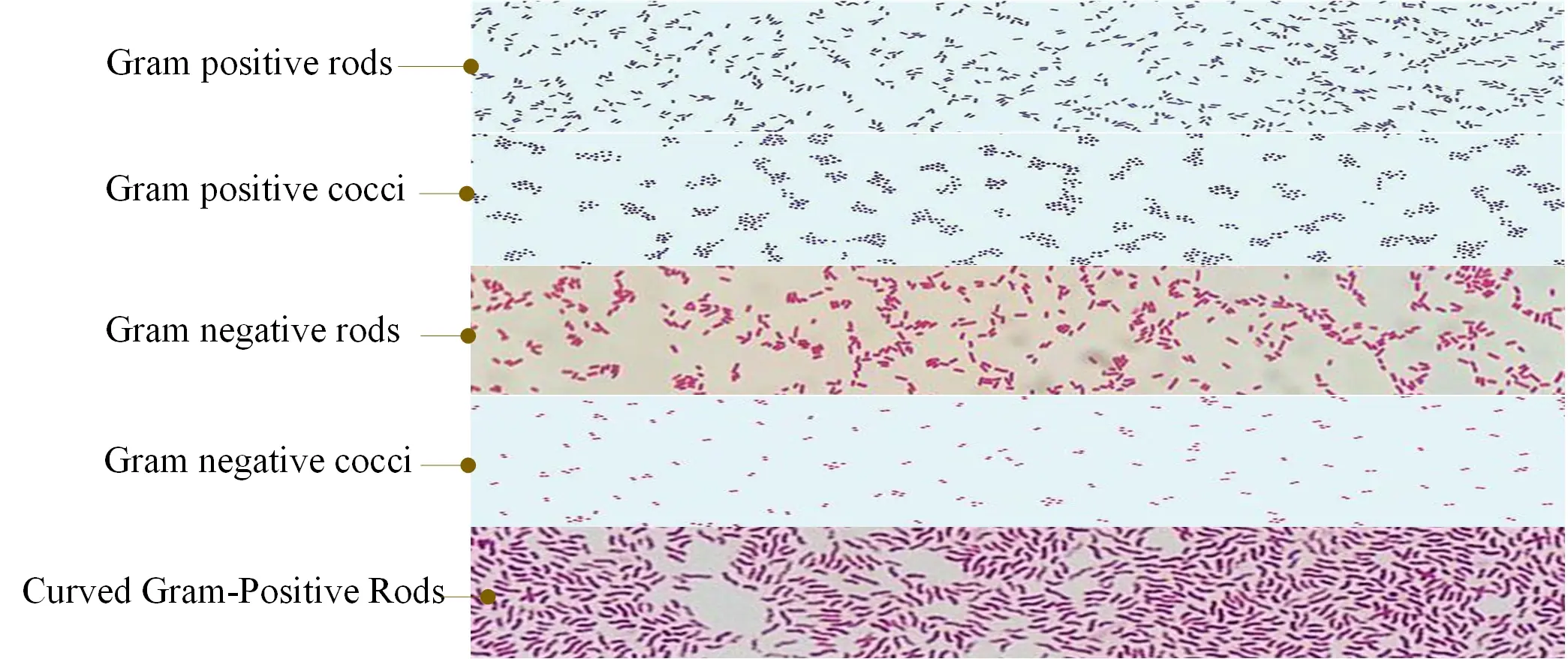
Gram stain results
Gram-negative bacteria will stain pink/red and Gram-positive bacteria will stain blue/purple.
It should be noted, however, that Gram staining can be variable for the same species (for example, pneumococcus discolors easily, likewise, certain Gram-positive bacteria when the colonies are old).
Some bacteria are even called “low gram” or “variable gram” (ex: corynebacteria).
☰ Risk of errors
Be careful, poor staining can lead to bacterial identification errors or poor diagnostic orientation.
False positive
- Spread too thick (bad discoloration of bacteria in depth),
- Dye deposit in the gentian violet bottle: filter the dye to remedy this
- Poorly drained iodine solution,
- Decolorizing agent left too little time,
- Use of fuchsin with certain germs: Neisseria and Acinetobacter, in particular, are "greedy" for fuchsin and concentrate it to a dark red that is difficult to distinguish from violet.
False negative
- In any population of Gram + we see Gram - (sometimes very numerous): these are corpses of Gram + bacteria,,
- Poor quality or too diluted dyes,
- Lugol's solution left for too little time,,
- Decolorizing agent left too long or poorly rinsed.
◉ Quality control
Spread a thin layer of stool on a slide and color it. There should appear many Gram positive and negative bacteria.
Note : It is recommended to carry out, with the same preparation of diluted stools, one or more dozens of slides which are dried, fixed and wrapped separately with aluminum foil. They are stored at +4°C. This eliminates the parameter: smear thickness and focuses on reagent quality and staining and destaining times.
◉ Comments and Tips
● If the loop is still hot when bacterial sample is obtained, cells will be destroyed
● Do not overheat or bacterial cell walls will be damaged and cell morphology will be destroyed.
● The thickness of the smear used will affect the staining result. The most crucial step to achieve the staining result is the decolorizing step.
● Excessive decolorizing will lead to a false result where Gram positive smears may appear pink or red indicating a Gram negative result, and insufficient decolorizing will lead to a false result where Gram negative smears may appear blue to purple indicating a Gram positive result.
● The degree of discoloration required is determined by the thickness of the smear.
● The (#ASM) group recommends that cells be prepared with a thin smear without areas of clumping or inconsistency. When staining thin smear, a short destaining time should be used.
● It is recommended to use young actively growing cultures. An intact cell wall is required for an accurate. Older cultures may have breaks in the cell wall and often give variable Gram results where a mixture of cells
● When viewing slides, use brightfield microscopy and adjust the brightness enough to reveal the color of the sample.
● For some author Gram stain was first described by Carl Friedlander, not by Christian Gram (Friedlander 1883)
◉ Gram-positive bacteria and Gram-negative bacteria
| Description of the Morphotype | Most Common Organisms |
|---|---|
| Gram positive cocci | |
| Pairs | Staphylococcus, Streptococcus, Enterococcus spp. |
| Tetrads | Micrococcus, Staphylococcus, Peptostreptococcus spp |
| Groups | Staphylococcus, Peptostreptococcus, Stomatococcus spp. |
| Chains | Streptococcus, Peptostreptococcus spp. |
| lancet-shaped | Streptococcus pneumoniae |
| Gram-negative cocci | |
| Neisseria spp., Moraxella catarrhalis. | |
| Gram-positive bacilli | |
| Small | Listeria monocytogenes, Corynebacterium spp. |
| Medium | Lactobacillus, bacilles anaérobies |
| Large | Clostridium, Bacillus spp. |
| Pleomorphic, Gram variables | Gardnerella vaginalis |
| Gram-negative coccobacilli | Bordetella, Haemophilus spp. (Pleomorph) |
| Gram-negative bacilli | |
| Small | Haemophilus, Legionella (thin with filaments), Actinobacillus, Bordetella, Brucella, Francisella, Pasteurella, Capnocytophaga, Prevotella, Eikenella spp. |
| Bipolar | Klebsiella pneumoniae, Pasteurella spp., Bacteroides spp. |
| Medium | Entériques, pseudomonas |
| Large | Clostridia or devitalized bacilli |
| Curved | Vibro, Campylobacter spp. |
| Spiral | Campylobacter, Helicobacter, Gastrobacillum, Borrelia, Leptospira, Treponema spp |
| Fusiform | Fusobacterium nucleatum |
| Filaments | Fusobacterium necrophorum (pleomorph) |
◉ Frequently Asked Questions
Q: Who is the author of the Gram stain?
A: The Gram stain owes its name to the Danish bacteriologist Hans Christian Gram (1853 – 1938) who developed the staining protocol in 1884.
Q: What are the 4 steps of Gram staining?
A: Gram staining involves staining the bacteria, fixing the color with a mordant, destaining the bacteria, and applying a counterstain.
For some authors, they consider that there are 5 stages of Gram staining, the first of which is the making of a smear of bacteria on a slide, then the staining of the bacteria, the fixation, the discoloration and finally the counterstaining.
Q: What are the 2 types of Gram stain?
R: Two major groups: gram-positive and gram-negative
Q: Why do we say that the Gram stain is a differential stain?
R: It is called a differential stain because it allows bacteria to be differentiated according to 2 main criteria: their shape (pairs, tetrads, groups, chains, etc.) and their affinity for dyes (: gram-positive and gram-negative).
Q: What color is gram negative?
A: Gram-negative organisms are pink-red in color.
Q: What color is gram positive?
R: Gram-positive organisms are purple or blue in color
Q: Why is iodine used in Gram stain?
A: The iodine is added as a mordant to bind the dye, it forms the crystal violet-iodine complex so that the dye cannot be removed.
Q: Why is alcohol used in Gram stain?
A: Gram-negative cell walls contain a high concentration of alcohol-soluble lipids. The bleach dissolves lipids, increasing cell wall permeability and allowing the crystal violet-iodine complex to flow out of the cell.