Summary :
Mueller Hinton Agar (MHA) is a common culture medium used for antimicrobial susceptibility testing (AST) of bacteria. Its composition provides optimal conditions for the growth of most clinically significant bacteria. In this article, we explore its principle, its preparation, its interpretation and its different uses.
◉ Overview
Mueller Hinton Agar is a standardized solid medium recommended for the study of the susceptibility of bacteria to antimicrobial agents by the method of diffusion (Kirby-Bauer method) or dilution in agar.
It was originally formulated by Mueller and Hinton as a protein-free medium for the primary isolation of Neisseria species.
This non-supplemented medium, initially recommended by Bauer et al, was selected by CLSI, CA-SFM and by EUCAST as the reference medium for several reasons.
This culture media is poor in sulfonamides, trimethoprim and tetracycline inhibitors, and allows satisfactory growth of most non-demanding pathogens while demonstrating batch-to-batch reproducibility.
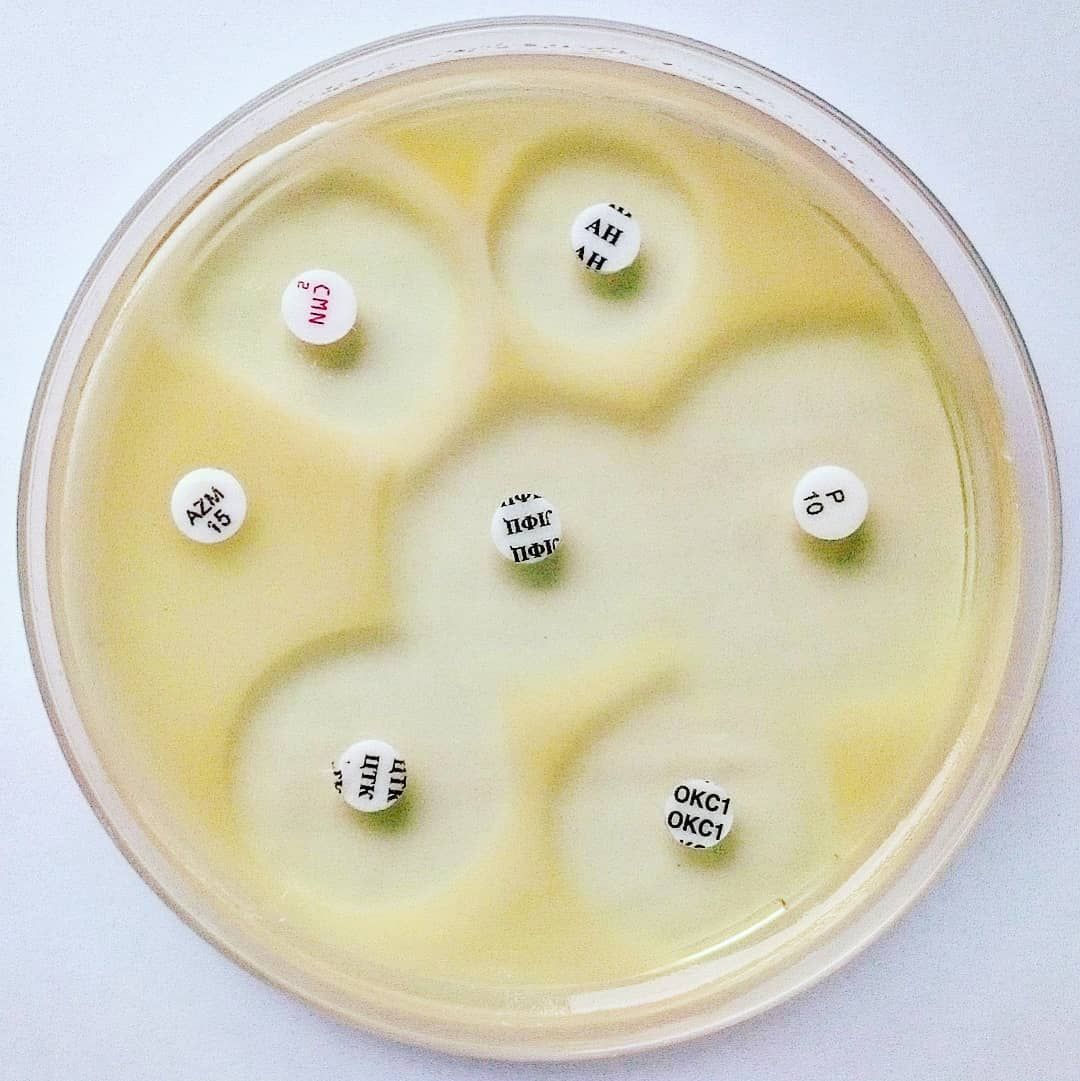
Mueller Hinton Agar
◉ Preparation and Composition of Mueller Hinton Agar
Suspend the components, dehydrated powder, in water (38 grams in 1000 ml of purified / distilled water). The medium is boiled for a few seconds until the ingredients are completely dissolved. Sterilize by autoclaving at 15 lbs (121 ° C) pressure for 15 minutes.
Cool to 47 ° C, mix well before pouring into sterile Petri dishes.
Mueller Hinton Agar Composition |
|||
|---|---|---|---|
| Ingredients | gram / liter | ||
| Peptone | 17.5g | ||
| Meat extract | 2g | ||
| Starch | 1.5g | ||
| Agar | 17g | ||
| Final pH | 7,3 +/- 0,1 | ||

Mueller Hinton Dehydrated
◈ A variety of supplements can be added to Mueller Hinton Agar , including 5% defibrinated sheep or horse blood, 1% growth supplement, and 2% sodium chloride.
◈ Starch is added to absorb all toxic metabolites produced
Mueller Hinton Agar's standardized composition and consistent performance make it an ideal medium for AST, enabling accurate and reliable interpretation of antimicrobial susceptibility results in clinical microbiology laboratories.
◉ Procedure
◈ A standardized suspension of the organism is buffered over the entire surface of the medium.
◈ Paper discs impregnated with specific amounts of antibiotics or other antimicrobial agents are placed on the surface backing.
◈ La boite est incubée et les zones d'inhibition autour de chaque disque sont mesurées. Détermination de la susceptibilité est faite en comparant la taille de zone obtenue aux tailles de zone dans CLSI, EUCAST
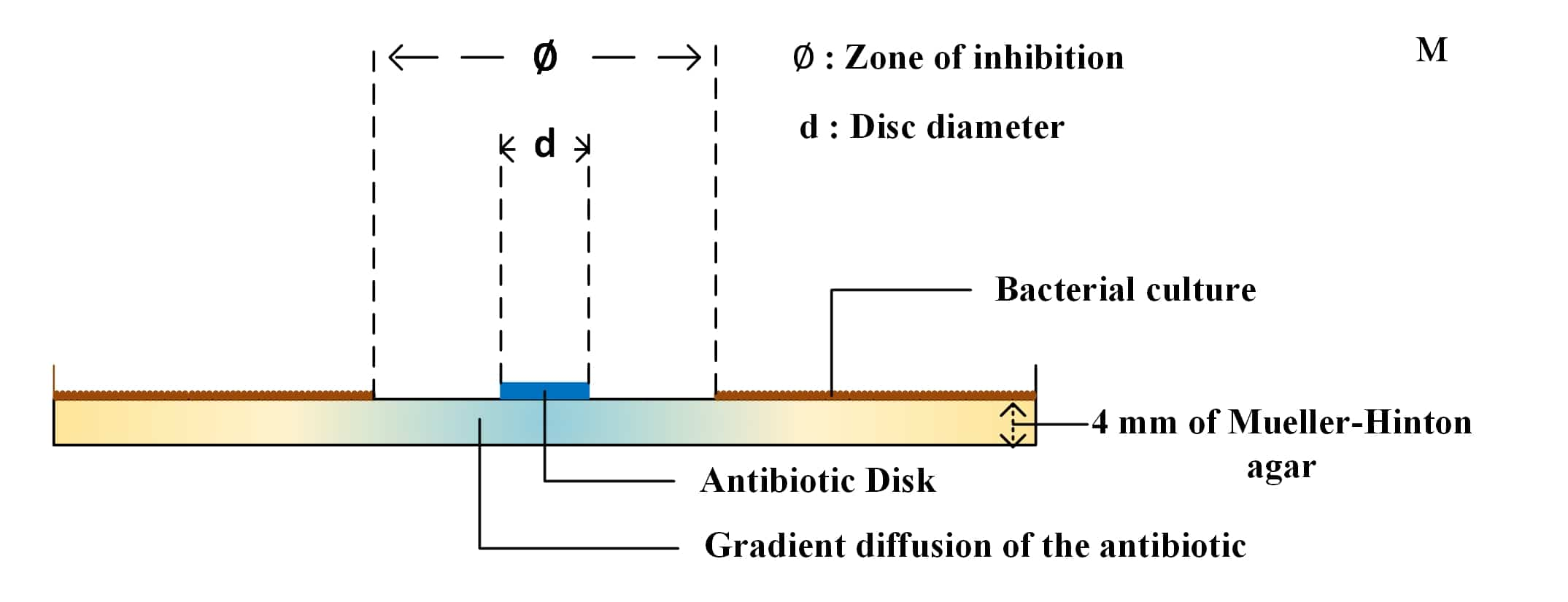
Antibiotic Susceptibility Testing

Pseudomonas on Mueller - Hinton
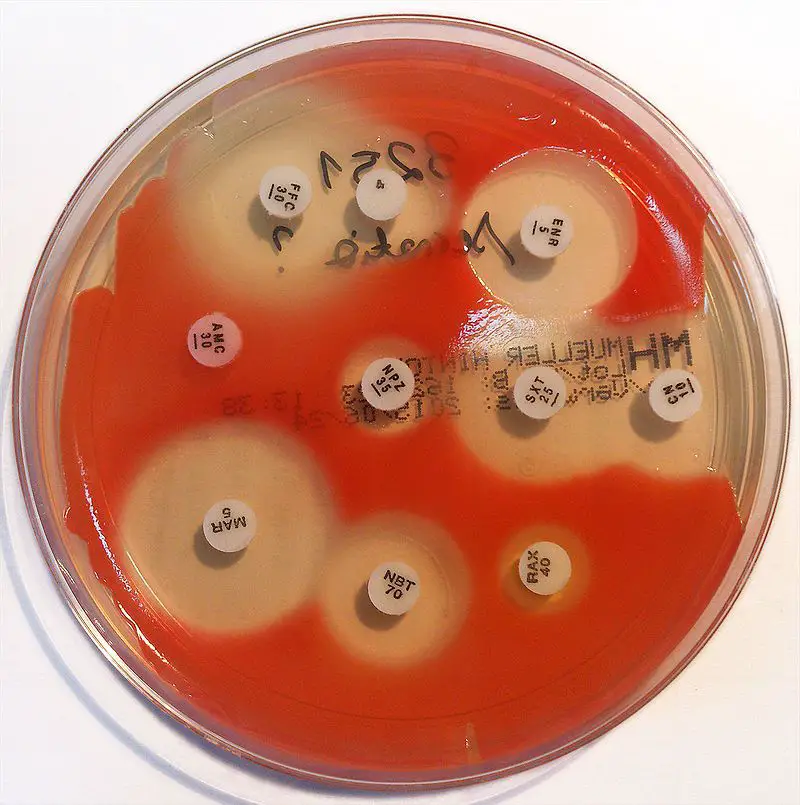
Serratia on Mueller - Hinton
Limits of use
Some bacteria may not thrive on HD media due to their nutritional requirements. It is then recommended to use an appropriate medium: HTM for Haemophilus, Mueller-Hinton supplemented with 5% sheep blood for Streptococcus pneumoniae and ß hemolytic streptococci, GC agar for Neisseria gonorrhoae. These recommendations are well described in the various CLSI, CA-SFM or EUCAST standards.