🏾 Content :
🏾 Sodium thiosulphate
Sodium thiosulfate is an inorganic sodium salt with the formula Na2S2O3 composed of a 2:1 mixture of sodium and thiosulfate ions
The uses of sodium thiosulfate are numerous, in particular as a fixing agent or to neutralize the effect of biocides such as dichlor, iodine and other oxidants, also, it has a role as an antidote to cyanide poisoning, nephroprotective agent and antifungal.
It is usually added to table salt at less than 0.1% and to alcoholic beverages at less than 0.0005%. It is usually available as an oral product without a prescription.
Sodium thiosulphate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| CAS number | 7772-98-7 | ||||||||
| Molecular mass | 158.108 g/Mol | ||||||||
| Formula | Na2S2O3 | ||||||||
| Synonyms | disodium thiosulfate, sodium hyposulfate, sodium thiosulfate pentahydrate. | ||||||||
| Appearance | White powder | ||||||||
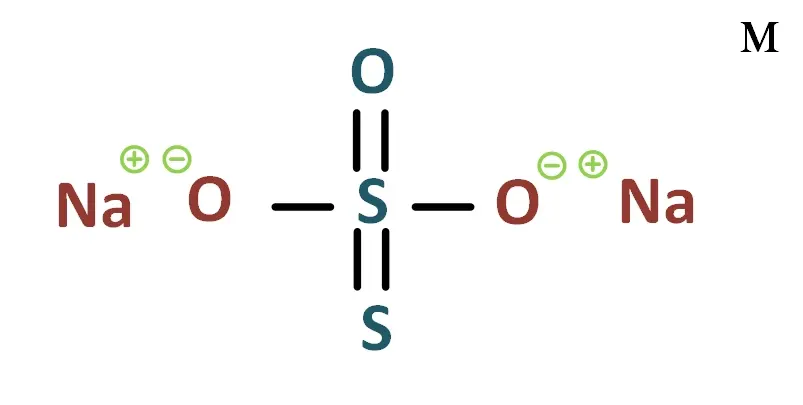
Thiosulfate de sodium : Na2S2O3
🏾 Applications and uses of sodium thiosulfate
The action of sodium thiosulfate is multifactorial since it is a chelating agent of divalent cations, which also has antioxidant and vasodilator properties.
- Sodium thiosulfate is used to treat cyanide poisoning. It is also used to reduce the side effects of the cancer drug cisplatin.
- It is used to treat, under certain conditions, calciphylaxis, kidney stones, uremic vascular calcification and coronary artery calcification.
- Sodium thiosulfate would promote the healing of skin ulcerations, especially when treatment with hyperbaric oxygen therapy is applied concurrently.
- It is added in small amounts to ammonium thiosulfate, which is used as a photographic fixing salt.
- Hydrated salt is used as an anti-chlorine in bleaching, it simply reacts and forms sodium hydrogen sulfate or sodium bisulfate which is an inactive salt.
🏾 Properties
Sodium thiosulfate is a colorless monoclinic crystal or white crystalline powder, odorless and salty. The relative density for this is 1.667. Water-soluble, its solubility at 100°C is 231 g/100 ml of vapour.
Sodium thiosulfate salt decomposes at high temperatures to give sodium sulfate with sodium polysulfide. It dissociates in water and some other polar solvents.
When exposed to dilute acids such as dilute hydrochloric acid, sodium thiosulfate salt undergoes a decomposition reaction to yield sulfur with sulfur dioxide.