🏾 Contenu :
🏾 Overview
◉ A pH meter is a device, often electronic, allowing the measurement of the pH (acidity or alkalinity) of a solution using a numerical scale from 1 to 14. It is most often made up of an electronic box giving the possibility of displaying the digital pH value and a pH probe.
In general, this probe is a combined electrode, i.e. it consists of two electrodes :
- One with a known and constant potential (called a reference electrode ).
- An indicator electrode, called a glass electrode, the potential of which varies with pH.
The potential between these two electrodes is zero at pH=7. The pH value can then be determined by correlation because the potential difference between the two electrodes changes in proportion to the pH.
Monitoring pH levels is an important step in many areas: the production of certain foods, the culture medium, chemical solutions, soil, quality control, etc.

pH meter
🏾 PH meter Parts
◉ A pH meter is a scientific instrument that measures the activity of hydrogen ions in water-based solutions, indicating its acidity or alkalinity expressed as pH. It consists of two basic elements (3 for some models):
1- A high input impedance meter This is the main body, which houses the microchip used to process minute electrode voltages and display measurements in pH units on a display.
2- The probe or the combined electrode : made up of 2 electrodes, this is the part where the measurement actually takes place. The probe is the consumable, sensitive and most expensive part of the meter; and should be handled with care. The combination electrode consists of a measuring electrode (glass electrode) and a reference electrode, both immersed in the same solution. In order to obtain a defined pH value, the reference electrode must have a defined stable potential which is independent of the measured solution.
- Reference electrode consists of a reference element (eg silver/silver chloride) which is immersed in a defined electrolyte. This electrolyte must: :
- Being in contact with the measured solution through, most often, a porous ceramic junction.
- Having a high concentration of ions which results in low electrical resistance.
- Sufficiently stable over a wide temperature range.
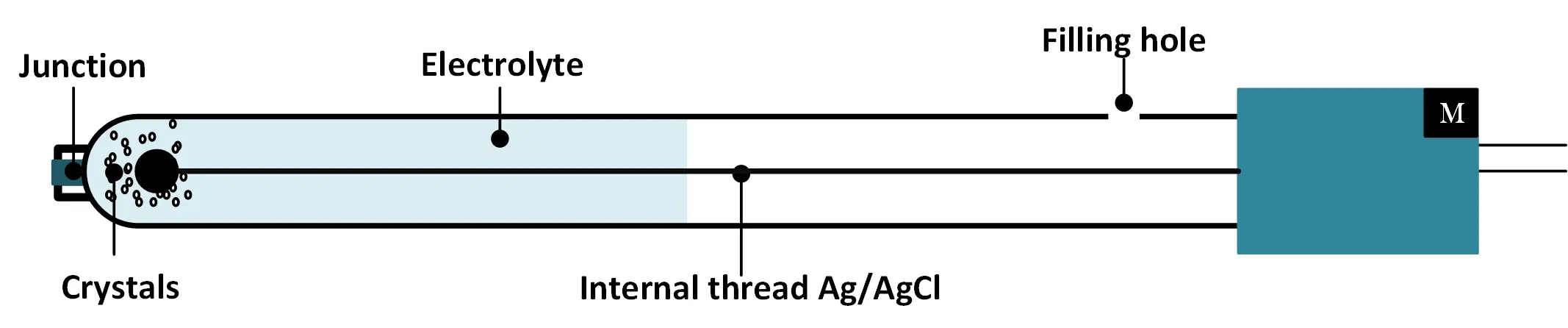
Reference electrode
- The pH glass electrode : different lengths and shapes are available to suit the most varied applications. It is a hydrogen ion sensitive glass bulb, with a millivolt output that varies with changes in the relative concentration of hydrogen ions inside and outside the bulb.
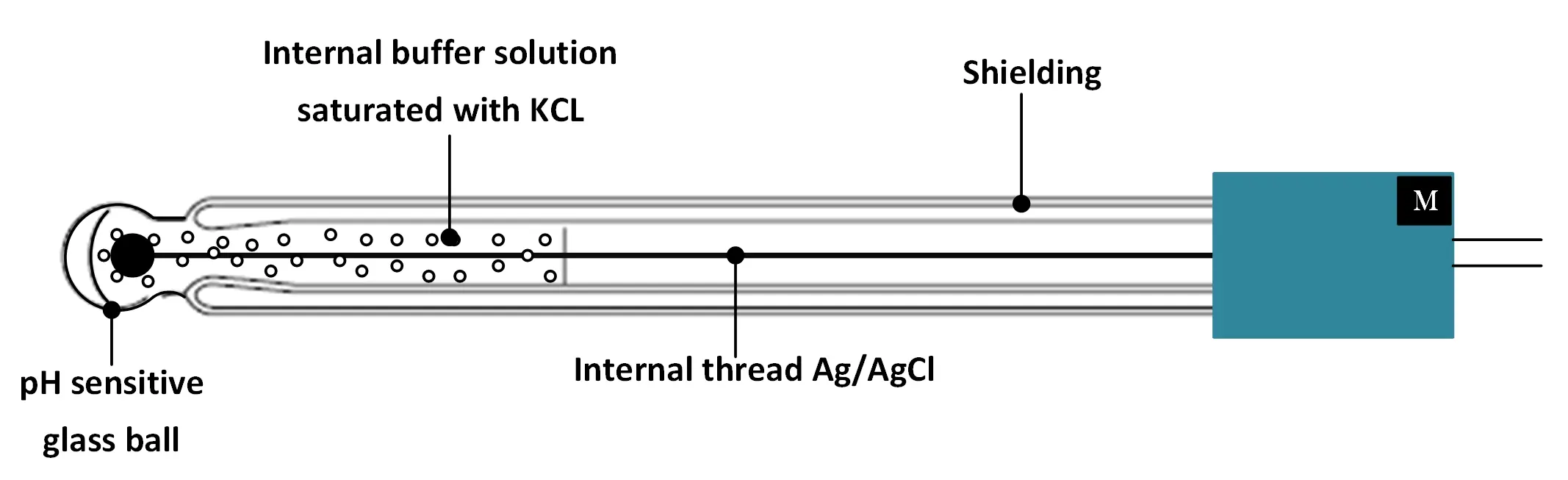
pH glass electrode
3- thermometer probe : Some pH meters are equipped with the ability to measure the temperature of the solution being measured and factor it into the reading that the meter displays (pH is directly influenced by the temperature of the solution). This function is called ATC, for "Automatic Temperature Compensation"..
🏾 Principle of pH meters
The principle of operation is based on measuring the change in electronic voltage potential when immersed in the solution under test by measuring the concentration of positively charged free hydrogen ions.
The glass electrode is filled with an electrolyte solution which reacts to the presence of hydrogen ions. The electrolyte does this by accepting or releasing electrons in an effort to keep the environment neutrally charged, with no more positively charged particles than negatively charged particles.
When this happens, the amount of electrons available in the solution changes. This change is what the meter measures and then extrapolates into the number you see on the meter display.
🏾 How to Calibrate a pH Meter ?
Allow all buffers to reach the same temperature, as pH readings are temperature dependent. Then pour approximately 30 mL each of pH 10.01, 7.00, and 4.01 buffers into separate 50 mL beakers. Use these three beakers as rinse beakers during calibration.
Rinse the pH electrode first with deionized water and then in the rinsing beaker with pH 10.01 buffer.
Place the electrode in the calibration beaker of pH 10.01 buffer, so that the electrode tip and junction are completely immersed in the buffer, and stir the buffer at a moderate, even speed.
Start calibration on the meter. Wait for a stable reading in pH 10.01 buffer, at least 1-2 minutes.
Once the correct buffer value has been entered, proceed to the next calibration point.
Repeat steps with 7.00 pH buffer and 4.01