Contenu :
Ⅰ. Overview
The Malachite green staining (Schaeffer-Fulton method ) is the most common method used to perform endospore staining. Malachite green stain can also used as a simple stain for bacterial cells.
The Schaeffer-Fulton method uses heat to push the primary dye (malachite green) into the endospore. Washing with water decolorizes the cell, but the endospore retains the green color. The cell is then counterstained pink with safranin.
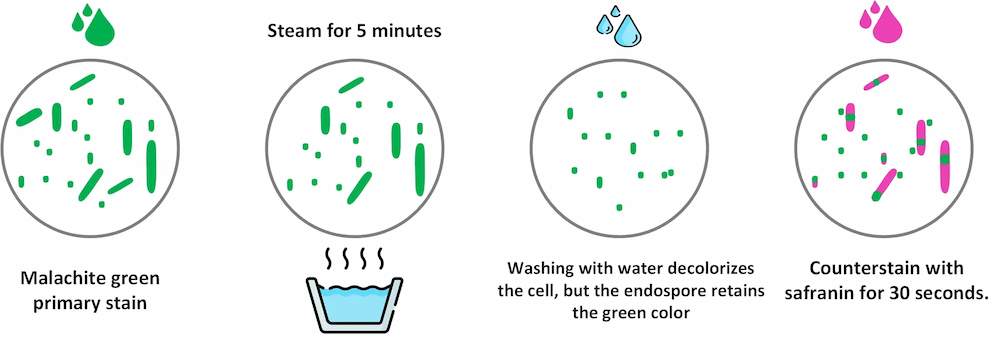
Malachite green staining
🏾 Endospores : Endospores are light green.
🏾 Vegetative cells : Vegetative cells are brownish-red to pink.
🏾 Examples of positive endospore staining : Clostridium perfringens, C. botulinum, C. tetani, Bacillus anthracis, Bacillus cereus, Desulfotomaculum spp, Sporolactobacillus spp, Sporosarcina spp,
Ⅱ. What is a bacterial endospore?
An endospore or spore is a tough structure produced by certain pathogenic bacterial genera such as Clostridium in order to survive in adverse environmental conditions. Endospores can reside inside the bacterial cell or can exist as free spores
Bacteria can remain in this sporulated state until conditions become favorable and they can germinate and return to their vegetative state.
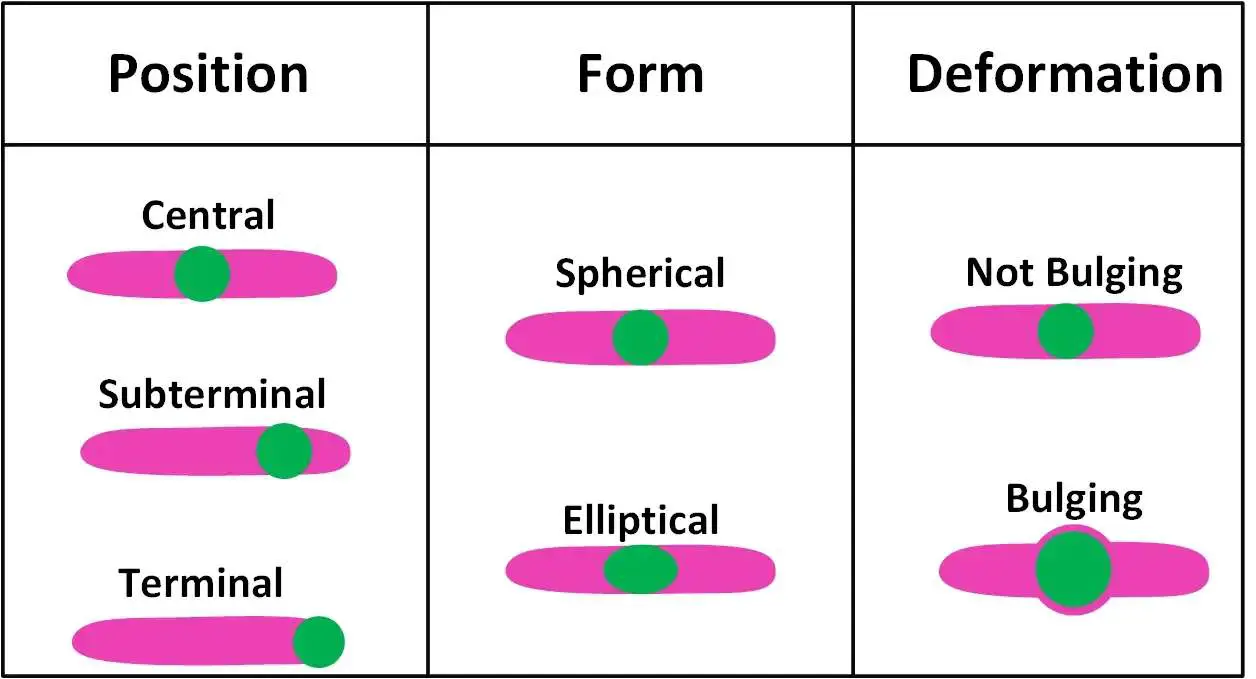
Shape and position of bacterial spores
Ⅲ. Principle of Endospore Staining
The primary dye Malachite green is a relatively weakly binding dye to the cell wall and spore wall. In fact, if washed well with water, the dye comes right out of the cell wall, however not from the spore wall once the dye is locked in.
Malachite green is forced into the spores by steaming the bacterial emulsion. This heating step stains the vegetative cells and the endospores. The water acts as a decolorizer for the vegetative cells, but the stain is not released by the endospores and free spores. The safranin counterstain is used on the slide to give color to the vegetative cells. The endospores will have retained the malachite green, appearing green (sometimes a little bluish), and the vegetative cells will be brownish-red or pinkish.
Ⅳ. Endospore staining procedure
- Prepare a smear on a clear, dry glass slide
- Allow it to air dry and fix it with gentle heat.
- Flood the slide with 1% w/v Malachite Green
- Allow the stain to be in contact with the smear for 2-3 minutes and heat the preparation for 3-6 minutes and then allow to cool.
- Wash in slow-running tap water.
- Counterstain with 0.5% aqueous safranin for 30 seconds.
- Wash with water, blot dry and examine under oil immersion objective.
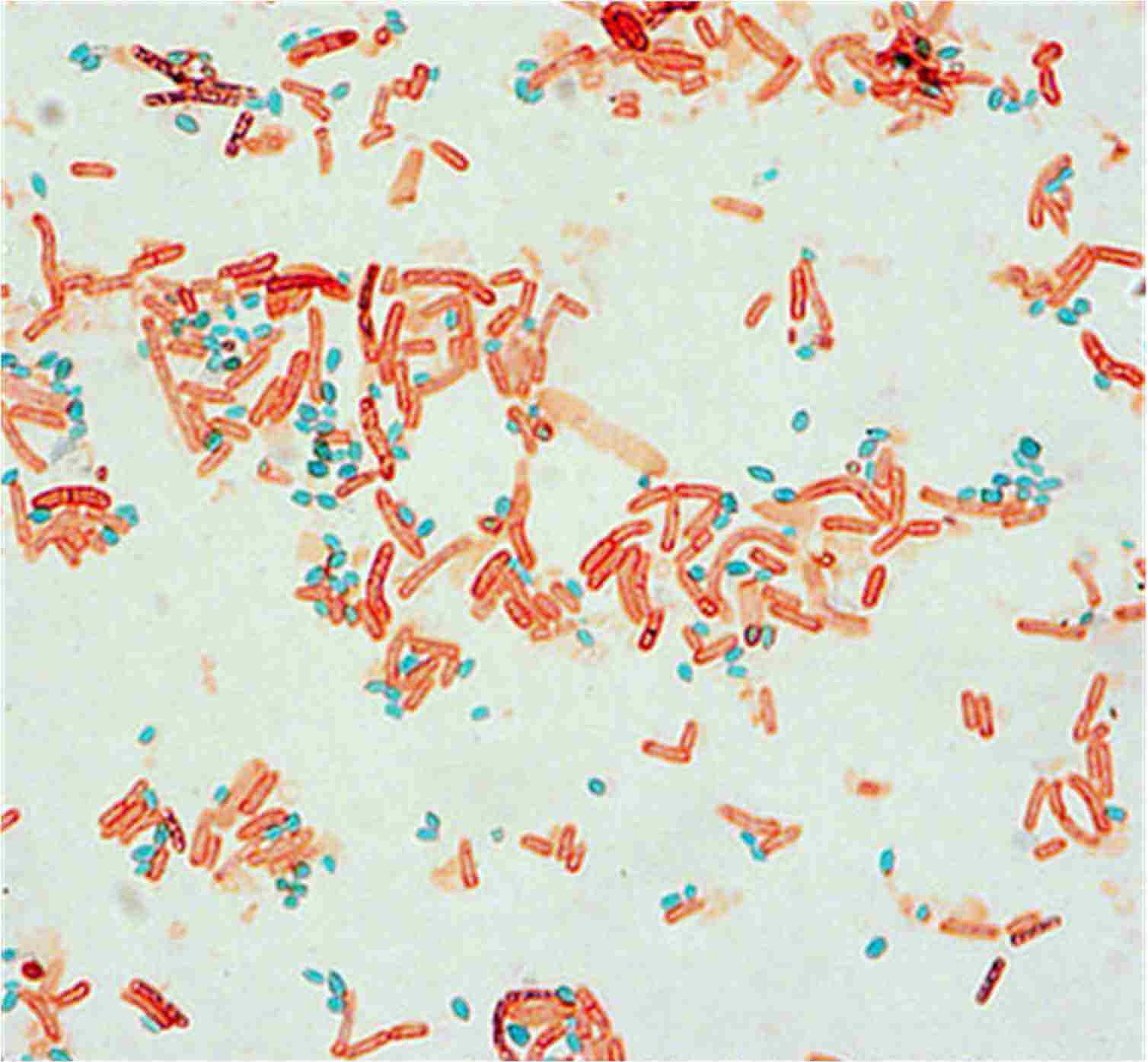
Endospore staining (source : @ASM)
🏾 Endospores : Endospores are light green.
🏾 Vegetative cells : Vegetative cells are brownish-red to pink.
🏾 Examples of positive endospore staining : Clostridium perfringens, C. botulinum, C. tetani, Bacillus anthracis, Bacillus cereus, Desulfotomaculum spp, Sporolactobacillus spp, Sporosarcina spp,
Ⅴ. Variation on Schaeffer-Fulton method
To prevent the release of harmful gases.
Cover the smear with malachite green for a minimum of 45 min. Wash it with water, wipe it with filter paper. Then cover the slide with a mercurochrome solution for two minutes. Wash it and dry it again. The smear is therefore ready to be observed under the microscope at the x100 objective with immersion oil.
Ⅵ. Comments and tips
1- Some labs do not use paper towels as described in the Schaefer-Fulton procedure (an ill-fitting piece of paper may burn or leak)
2- The age of the culture will affect sporulation. Young cultures (less than or a day old) may have only vegetative cells, whereas older cultures (5 to 7 days old) are excellent for good sporulation.
3- Heat fixing should be done with minimal flaming as excess heat will destroy the integrity of the cells, causing them to shrink and to aggregate together on the slide.
4- It should be noted that any debris on the slide can also take up and hold the malachite green stain and so caution should be taken when interpreting slides.
References:
- A Oktari - The Bacterial Endospore Stain on Schaeffer Fulton using Variation of Methylene Blue Solution
- Staining procedures - UK Standards for Microbiology Investigations
- ASM - Endospore Stain Protocol
- OpenStax - Microbiology
- Himedia - Malachite Green 1% w/v
- UW university - ENDOSPORE STAIN
- Libretexs - Spore Stain
- techmicrobio.eu - LA COLORATION DES SPORES